Option D: 246 K
According to ideal gas law:
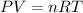 ...... (1)
...... (1)
Here, P is pressure, V is volume, n is number of moles, R is Universal gas constant and T is temperature of the gas. The value of R is
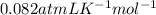 .
.
Number of moles of gas are 0.750 mol, volume of cylinder is 6850 mL and pressure of gas is 2.21 atm.
First converting volume from mL to L
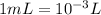
Thus,
6850 mL=6.850 L
Putting the values in equation (1),
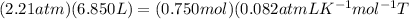
Rearranging
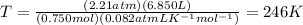 ,
,
Therefore, temperature of the gas is 246 K.