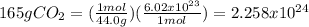you can't convert grams to molecules directly. First, we need to convert the grams to moles, and then to molecules
to convert grams to moles, we use the molar mass of the molecule. to convert moles to molecules, we use Avogadro's number. (1 mol= 6.02 x 10²³ molecules)
molar mass of CO₂= 12.0 + (2 x 16.0)= 44.0 g/mol