Answer: 0.47 moles
Step-by-step explanation:
Initial moles of
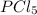 = 1 mole
= 1 mole
Volume of container =
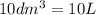
Initial concentration of

The given balanced equilibrium reaction is,
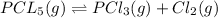
Initial conc. 0.1 M 0 0
At eqm. conc. (0.1-x) M (x) M (x) M
The expression for equilibrium constant for this reaction will be,
![K_c=([PCl_3][Cl_2])/([PCl_5])](https://img.qammunity.org/2018/formulas/chemistry/college/2799kiwimpt012f3cntix2myjkwah0c74k.png)
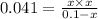
Solving for x:
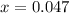
Thus concentration of
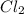 produced is 0.047 M
produced is 0.047 M
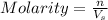
where,
n= moles of solute
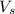 = volume of solution in L
= volume of solution in L
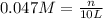

Thus moles of
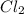 produced is 0.47.
produced is 0.47.