Answer:
The correct answer is option B.
Step-by-step explanation:
Enthalpy of the system =
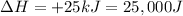
Entropy of the system =
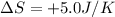
Temperature of the system =T =23°C = 296 K
The Gibbs's free energy is given by;
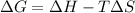
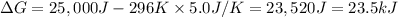
The value of ΔG is positive which means that system is not spontaneous.
Hence, from the given options the most closest value to our answer is option B.