Answer:
Option (a) is correct.
The final volume of a gas is 112.5 mL.
Explanation:
Given : Initial volume of gas = 75 mL
And the pressure decreases from 300 kPa to 200 kPa.
We have to determine the final volume of a gas.
Using Boyle's Law:
The Volume of an ideal gas is inversely proportional to its pressure.
That is
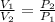
That is
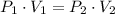
Given:
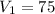 ml
ml
 KPa
KPa
and
 KPa
KPa
Let
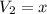 ml
ml
Thus,
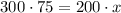
Divide both side by 200, we have,
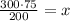
Simplify, we have,
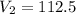 ml
ml
Thus, The final volume of a gas is 112.5 mL.