Answer:
The correct answer is calcium hydroxide,
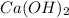 .
.
Step-by-step explanation:
Acids are neutralize by alkaline that basic solution or by base. So,in order to neutralize nitric acid we will need an alkaline solution.
From the given options the alkaline solution is of calcium hydroxide.

Where as hydrochloric acid is an acid, water is neutral liquid and lemon juice is also an acidic solution which contains citric acid.
The correct answer is calcium hydroxide.