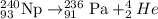Answer : The balanced alpha decay reaction will be,
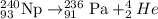
Explanation :
Alpha decay : In this process, alpha particles is emitted when a heavier nuclei decays into lighter nuclei. The alpha particle released has a charge of +2 units.
The general representation of alpha decay reaction is:
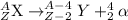
The given nuclear reaction is:
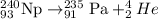
This reaction is an alpha decay reaction and this nuclear equation is an unbalanced reaction because in this reaction the atomic mass of 'Pa' should be 236 instead of 235.
Hence, the balanced alpha decay reaction will be,