Answer: Option (d) is the correct answer.
Explanation:
It is known that elements of group 17 are halides. These include fluorine, chlorine, bromine, iodine and astatine.
Therefore, a molecule that includes any halide group along with hydrocarbon is known as a halide molecule.
Thus,we can conclude that out of the following options
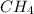 is not a halide hydrocarbon.
is not a halide hydrocarbon.