Answer: The correct answer is 1.43 cal.
Step-by-step explanation:
The expression of heat in terms of mass and temperature is as follows;
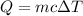
Here, Q is the heat, m is the mass of the object and
 is the change in temperature.
is the change in temperature.
It is given in the problem that 1.25 grams of silver is cooled from 100.0°C to 80.0°C.
Calculate the change in temperature.
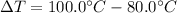
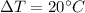
Calculate the heat in calories.
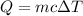
Put m= 1.25 grams,
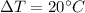 and c=0.057 cal/g°C.
and c=0.057 cal/g°C.
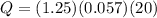
Q= 1.43 cal
Therefore, the amount of heat is 1.43 cal.