Answer:
c.32
Step-by-step explanation:
Hello,
In this case, it is possible to determine it by building the subshells organization for the first four shells as shown below:
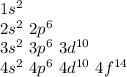
In such a way, counting the electrons at the n=4 shell, the result is 32 (2+6+10+14), so the answer is c.
Best regards.