Answer:
D.
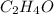
Step-by-step explanation:
For this case, we have to keep in mind that the structure of the aldehyde has an oxo group (C=O) bonded to a primary carbon. Therefore we have to search for this group in the options given.
For the first option, we have an OH group, so it is an alcohol. For B. we have an acid because the carboxylic acid group is present (COOH). For C. we have a very complex structure in which we have several oxo groups but neither is bonded to a primary carbon. In the last structure, we have the aldehyde group
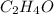 (see figure).
(see figure).