Answer: The equation is given below.
Step-by-step explanation:
Calcium phosphate does not dissolve in water but in acid it does.Similarly cobalt phosphate is also soluble in acidic solution as it get dispersed in the acid solution.
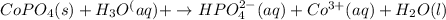
When cobalt phosphate dissolves in acidic solution as it get strongly attracted by the acid molecules and dissociates into cobalt(III) ions, hydrogen phosphate and water molecule.
Hence, the net ionic equation for cobalt phosphate in hydronium ion is given above.