Step-by-step explanation:
It is known that metals are the species which easily lose an electron to acquire stability.
For example, atomic number of magnesium is 12 and its electronic distribution is 2, 8, 2.
Hence, in order to attain stability it will readily lose two electrons. As a result, it will form
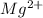 ions.
ions.
Thus, we can conclude that 2 electrons must be lost by the element Mg.