Answer:
Ionic strength
Step-by-step explanation:
Ionic strength is an indicator or a measure of the concentration of ions in a solution. A solution with a high ionic strength implies a higher concentration of ions which suggests the presence of a greater number of ions.
Ionic strength ( I) is calculated using the Debye-Huckel formula:
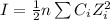
where n = number of ions
Ci = concentration of the ith ion
Zi = charge on the ith ion