Answer: The correct answer is PbS.
Step-by-step explanation:
When lead acetate reacts with sodium sulfide, it leads to the formation of some products.
The chemical equation for the reaction of lead acetate and sodium sulfide, it follows:
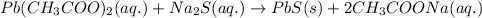
By Stoichiometry of the reaction:
1 mole of lead acetate reacts with 1 mole of sodium sulfide to produce 1 mole of black color of solid known as lead sulfide and 2 moles of sodium acetate.
Hence, the correct answer is PbS.