Answer: Option (d) is the correct answer.
Step-by-step explanation:
pH is defined as the amount of hydrogen ions present in a solution. It is also the negative log of concentration of hydrogen ion.
Mathematically, pH =
![-log [H^(+)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/yhuny7f00oxtjxsdmnkts3zwu0fowf3edy.png)
where,
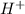 means hydrogen ions and
means hydrogen ions and
![[H^(+)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/tixcvmiul05vtrn8s7qstptt69zx1u6brh.png) is the concentration of sodium ions.
is the concentration of sodium ions.
When pH of a solution ranges from 0 to 6.9 then it means that solution is acidic in nature. When pH equals to 7 then solution is neutral in nature and when pH ranges from 7.1 to 14 then it means solution is basic in nature.
Thus, we can conclude that pH measures the concentration of hydrogen ions in solution.