Answer:
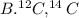
Step-by-step explanation:
Isotopes are atoms of the same element that have the same number of protons (needless to say since they are from the same element) but they have different numbers of neutrons and hence masses.
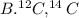
These are two different atoms from the same element, carbon with the same number of protons but different number of neutrons hence the different relative atomic masses