Answer : The moles of oxygen are, 9.6 moles.
Explanation : Given,
Moles of
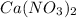 = 1.6 mole
= 1.6 mole
As we know that,
In
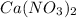 , there are 2 atoms of nitrogen, 1 atom of calcium and 6 atom of oxygen.
, there are 2 atoms of nitrogen, 1 atom of calcium and 6 atom of oxygen.
Now we have to calculate the moles of oxygen.
As, 1 mole of
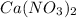 contains 6 moles of oxygen.
contains 6 moles of oxygen.
So, 1.6 moles of
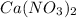 contains
contains
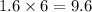 moles of oxygen.
moles of oxygen.
Therefore, the moles of oxygen are, 9.6 moles.