Answer:
The molecular formula is C₁₁H₂₂O₁₁.
Step-by-step explanation:
The empirical formula is the simplest expression to represent a chemical compound and indicates the elements that are present and the minimum proportion in integers between its atoms.
The molecular formula is the chemical formula that indicates the number and type of different atoms present in the molecule. The molecular formula is the actual number of atoms that make up a molecule.
The molecular formula is obtained, in this case, as (CH₂O)ₙ where n is a parameter that indicates how many times the empirical weight is repeated in the true molecule. This number of times must be an integer. If it is not, you must round it. This parameter n is determined by dividing the molecular mass of the compound by the molecular mass of the empirical formula.
Then, you know:
- C: 12 grams per mole
- H: 1 gram per mole
- O: 16 grams per mole
So
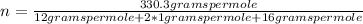
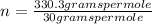
n=11.01
n≅11
So (CH₂O)₁₁. Now the subscripts of the empirical formula are multiplied by the proportion "n" to obtain the molecular formula.
So the molecular formula is C₁₁H₂₂O₁₁.