Data:
M (Molar Mass) of C2H6
C = 2*12 = 24 amu
H = 6*1 = 6 amu
---------------------
MM C2H6 = 24+6 = 30 g/mol
P (pressure) = 1.6 atm
V (volume) = 12.7 L
R = 0,082 atm .L/mol.K
n (Number of mols) →

Since
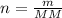 , we can perform the following substitution in the above Clapeyron equation:
, we can perform the following substitution in the above Clapeyron equation:
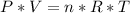
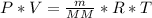
multiply cross
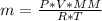
Solving:
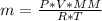
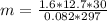
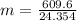
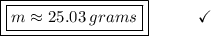
Answer:
25.03 grams of ethane gas