Answer:
You would use cyclohexane
Step-by-step explanation:
Hi, zinc chloride (
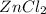 ) is soluble in water or other polar solvents, and the zinc is reactive in solvents with a oxidation potencial.
) is soluble in water or other polar solvents, and the zinc is reactive in solvents with a oxidation potencial.
This is why to determine the density of this salt you need to use a non-polar non-reactive solvent like cyclohexane, so the salt will remain intact and you can measure the volume that it displaced.
Knowing that volume and the weight you can determine the density.