Answer:
The volume of 425 g of ice, if the density of the ice is
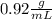 is 461.96 mL=0.46 L
is 461.96 mL=0.46 L
Step-by-step explanation:
The equation to find the volume is:
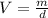 (1)
(1)
Where:
V: Volume
m: Mass
d: Density
We know the mass of ice (425 g) and the density (
![0.92(g)/(mL)). With this information we can replace in the eq (1) and we will find the volume</p><p>[tex]V=(m)/(d)](https://img.qammunity.org/2018/formulas/chemistry/high-school/rt7ry5s01up7gdjpkf4wcae0xy3ocv0hhf.png)
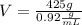
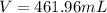
If we want to know the volume in liters, we know 1L=1000mL
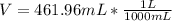
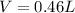
The volume is 461.96 mL or 0.46 L