Answer: Potassium has 1 valence electron.
Step-by-step explanation:
Valence electrons are defined as the electrons which are present in the last shell of the atom. They are identified by the electronic configuration of atom.
Sodium is the 11th element of the periodic table. It belongs to Group 1 and period 3. Electronic configuration of this element is
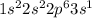
Sodium has 1 electron in the last shell. Thus, it has 1 valence electron.
Potassium is the 19th element of the periodic table. It belongs to Group 1 and period 4. Electronic configuration of this element is
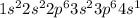
Potassium has 1 electron in the last shell.
Thus, it has 1 valence electron.