Answer: A) hydrogen bonding, B) dipole-dipole forces, C) London dispersion forces and D) London dispersion forces.
Step-by-step explanation: Methanol is a polar covalent molecule means it has dipole-dipole forces of attraction. Since, hydrogen is bonded to more electron negative oxygen atom, this molecule has hydrogen bonding. So, strongest attractive forces for this molecule are hydrogen bonding.
CO is a polar covalent molecule and so it has dipole-dipole forces of attraction.
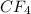 has four polar covalent bonds but the over all molecule is nonpolar as the dipole moment of one C-F bond is canceled by its opposite C-F bond as the molecule is tetrahedral. Being nonpolar, the molecule has london dispersion forces only.
has four polar covalent bonds but the over all molecule is nonpolar as the dipole moment of one C-F bond is canceled by its opposite C-F bond as the molecule is tetrahedral. Being nonpolar, the molecule has london dispersion forces only.
Ethane is also a nonpolar molecule and so it has london dispersion forces.