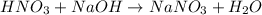Answer:
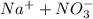
Explanation:
It is double displacement reaction in which exchange of ions take place.
When an acid and base combines to give salt and water, the double displacement reaction is called as neutralization. the nitric acid is a strong acid which dissociates to give
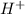 and
and
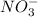 ions an d sodium hydroxide is a strong base which dissociates to give
ions an d sodium hydroxide is a strong base which dissociates to give
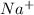 and
and
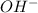 ions.
ions.
They result into formation of sodium nitrate which dissociates in water to give
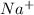 and
and
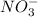 ions.
ions.