Answer: Temperature will increase the average kinetic energy of the colliding particles.
Step-by-step explanation:
Average kinetic energy is defined as the average of the kinetic energies of the particles present in a sample which are in random motion. Temperature is the measure of average kinetic energy of the particles.
Formula to calculate average kinetic energy is given by:
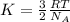
where,
K = kinetic energy
R = Gas constant
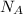 = Avogadro's constant
= Avogadro's constant
T = temperature
From above, it is clearly visible that kinetic energy is directly proportional to the average kinetic energy. As, the temperature increases, the average kinetic energy of the particles also increases and vice-versa.
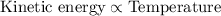
Hence, temperature will increase the average kinetic energy of the colliding particles.