Answer: The solid precipitate formed in the above reaction is silver chloride.
Step-by-step explanation:
Precipitation reaction is defined as the reaction in which an insoluble salt is formed when two solutions are mixed containing soluble substances. The insoluble salt settles down at the bottom of the reaction mixture.
Double displacement reaction is defined as the reaction in which exchange of ions takes place.
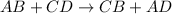
The chemical equation for the reaction of silver nitrate and potassium chloride follows:
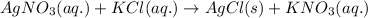
By Stoichiometry of the reaction:
1 mole of aqueous solution of silver nitrate reacts with 1 mole of aqueous solution of potassium chloride to produce 1 mole of solid silver chloride and 1 mole of aqueous solution of potassium nitrate
Hence, the solid precipitate formed in the above reaction is silver chloride.