Answer:
here since temperature and mass is same so aluminium must have more thermal energy than gold
Step-by-step explanation:
As we know that thermal energy stored inside the matter is given as
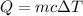
now we know that
m = mass of the given substance
c = molar specific heat capacity
 = temperature with respect to zero degree C
= temperature with respect to zero degree C
now we know that
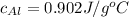 = molar specific heat capacity of aluminium
= molar specific heat capacity of aluminium
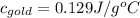 = molar specific heat capacity of gold
= molar specific heat capacity of gold
So here since temperature and mass is same so aluminium must have more thermal energy than gold