Answer:
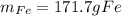
Step-by-step explanation:
Hello!
In this case, since we have 245 of iron (III) oxide, we first need to compute the moles contained there:
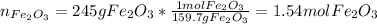
Now, as 1 mole of iron (III) oxide is related to 2 moles of iron, due to iron's subscript in the molecule, we get the moles of iron itself:
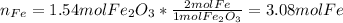
And the mass is computed based on the atomic mass of iron:
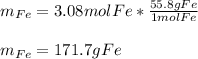
Best regards!