Answer: The correct option is (1).
Step-by-step explanation:
Whenever an atom in an excited state returns back to its ground state the energy is emitted out in the form of the radiation and the value of energy emitted can be determined by using the expression:
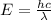
Where E = Energy of the radiation in joules
h = Planck's constant =
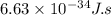
 = Wavelength of the radiation in meters.
= Wavelength of the radiation in meters.