Answer : The correct option is, 4.0 mole
Explanation : Given,
Moles of lithium = 12 mole
The given balanced chemical reaction is:

By the stoichiometry we can say that, 6 moles of Li react with 1 mole of
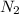 to give 2 moles of
to give 2 moles of
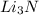
As, 6 moles of lithium react to give 2 moles of lithium nitride
So, 12 moles of lithium react to give
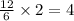 moles of lithium nitride
moles of lithium nitride
Therefore, the number of moles of lithium nitride produced will be 4.0 mole.