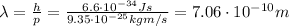First we need to convert the electron's energy in Joule. Keeping in mind that
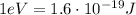
3 eV corresponds to
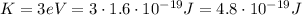
The kinetic energy of an electron is related to its momentum by the formula

where p is the electron's momentum, while m is its mass. Re-arranging the formula, we find
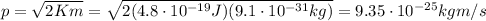
And then we can use De Broglie relationship to find its wavelength: