General principle of solubility is 'like dissolves like'
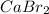
is an ionic compound, wherein the constituent ions (
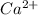
and
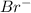
) are held by electrostatic forces of interaction.
Such ionic compounds are soluble in polar solvents.
Among the solvent mentioned in question, water (

) has maximum polarity. Hence,
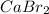
is most likely to dissolve in
