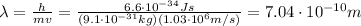First, we need to convert electron's kinetic energy into Joules. Keeping in mind that
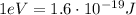
we have
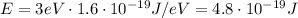
The kinetic energy of the electron is equal to:
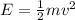
where m is the electron mass and v its speed. If we re-arrange this equation, we can find the electron speed:
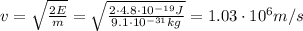
And now we can use De Broglie's relationship to find the electron's wavelength:
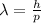
where h is the Planck constant and p=mv is the electron momentum. Substituting numbers, we get