Step-by-step explanation:
The given data is as follows.
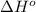 = 226.7 kJ/K mol,
= 226.7 kJ/K mol,
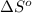 = 58.8 J/mol =
= 58.8 J/mol =
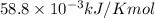
T =
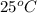 = (25 + 273) K = 298 K
= (25 + 273) K = 298 K
Now, calculate the standard free energy of formation of acetylene (
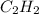 ) as follows.
) as follows.
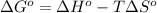
= 226.7 - kJ/mol - 298 K \times 58.8 \times 10^{-3} kJ/K mol[/tex]
= 209.2 kJ/mol
Thus, we can conclude that the standard free energy of formation of acetylene is 209.2 kJ/mol.