The solubility of NaCl at 30°C is 36.3 g/100 g of water. 108.9 g of NaCl is contained in a saturated solution that contains 300.0 grams of water
Explanation:
Usually, the term solubility refers to the quantity of solute which dissolves in a particular amount of solvent. Some of the substances that are soluble in aqueous are salts of Group IA, ammonium salts, acetates, nitrates.
Some salts might be insoluble in liquid and we call then as precipitates.
The solubility of NaCl at 30°C is 36.3 g, 100 g of water which means about 36.3 g of NaCl can be dissolved in 100g of water at 30°C
So, at the same temperature that is 30°C the number of grams of NaCl that can dissolve in 300g is ,
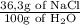 =
=
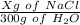
X g of NaCl =
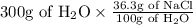
Therefore, X g of NaCl = 108.9 g of NaCl