number of moles of an element present can be calculated by dividing the mass present by the molar mass
the mass present is 10.0 g of each of the following elements
Na C Pb Cu Ne
molar mass 23 g/mol 12 g/mol 207.2 g/mol 63.5 g/mol 20.17 g/mol
number of moles = 0.43 =0.83 = 0.048 = 0.157 = 0.4958
-
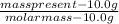
from the calculated number of moles, Pb contains the smallest number of moles.
since mass present is constant for all the elements, the number of moles was inversely proportional to molar mass, therefore Pb has the highest molar mass hence the least number of moles