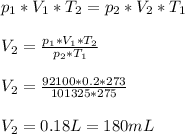To solve this question we will use ideal gas equation:
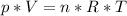
Where:
p = pressure
V = volume
n = number of moles
R = gas constant
T = temperature
We can rearrange formula to get:
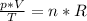
We are working woth same gas so we can write following formula. Index 1 stands for conditions before change and index 2 stands for conditions after change.

We are given:
p1=92.1kPa = 92100Pa
V1=200mL = 0.2L
T1=275K
p2= 101325Pa
T2=273K
V2=?
We start by rearranging formula for V2. After that we can insert numbers: