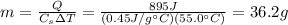The amount of heat Q needed to increase the temperature of a substance by

is given by
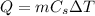
where
m is the mass of the substance
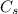
is the specific heat capacity of the substance

is the increase of temperature
For iron, the specific heat capacity is

. The amount of heat supplied to this sample is Q=895 J and the temperature increases by

, so we can re-arrange the previous formula to find the mass of the sample: