Step-by-step explanation:
Reduction means the gain of electrons or decrease in number of oxidation state of an atom.
For example,
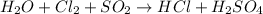
Reduction half reaction:
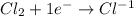
Here oxidation state of chlorine changes from 0 to -1, that is, chlorine is reducing in this chemical reaction.