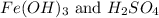Answer: The correct pair is,
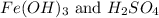
Explanation :
(1)
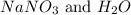
The balanced chemical reaction will be,
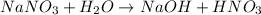
(2)
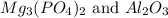
The balanced chemical reaction will be,
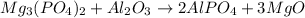
(3)
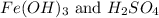
The balanced chemical reaction will be,
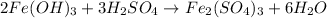
(4)
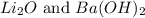
The balanced chemical reaction will be,
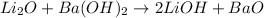
Hence, the pairs of reactants react together to produce water as one of the product will be,