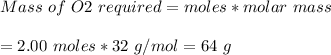Answer:
B) Mass of O2 required = 64.0 g
Step-by-step explanation:
Given:
Moles of Mg = 4.00 moles
To determine:
Mass of O2 that reacts completely with 4 moles of Mg
Step-by-step explanation:
The given reaction is:
2Mg + O2 → 2MgO
Based on the reaction stoichiometry:
2 moles of Mg reacts with 1 mole of O2
Therefore, 4 moles of Mg will need:
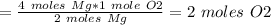
Molar mass of O2 = 32 g/mol