Answer:- 11.4 g of
 are produced by the combustion of 148 g of acetylene.
are produced by the combustion of 148 g of acetylene.
Solution:- Acetylene is a hydrocarbon that gives carbon dioxide on combustion in presence of oxygen. The balanced equation for the combustion of acetylene is:
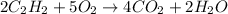
From balanced equation, there is 2:4 mol ratio between acetylene and carbon dioxide.
First of all we convert grams of acetylene to moles on dividing it's grams by molar mass of it. Then in next step, we multiply by mol ratio to get the moles of carbon dioxide.
Molar mass of acetylene is 26 . The set up would be as:
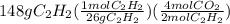
= 11.4 mol

So, 11.4 moles of
 are produced by the combustion of 148 g of acetylene.
are produced by the combustion of 148 g of acetylene.