Answer: Sublevel designation is 4d.
The allowable
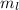 values are -2, -1, 0, +1, +2
values are -2, -1, 0, +1, +2
Number of orbitals in 4d sublevel are 5.
Step-by-step explanation: Principle Quantum number or 'n' tells us about the number of orbits.
Azimuthal Quantum number or 'l' tells us about the sub-levels. It is calculated by the formula
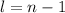
Magnetic quantum number of
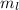 tells us about the number of orbitals. Its value ranges from
tells us about the number of orbitals. Its value ranges from
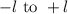
When n = 4, value of l = 0, 1, 2, 3 which designated s, p, d and f sub-level respectively.
Sub-level designation for the given values is 4d.

Number of orbitals = 5