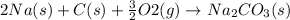Step-by-step explanation:
A balanced equation is defined as the equation which contains same number of atoms on both reactant and product side.
Chemical formula of sodium carbonate is
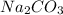 and it is formed by chemical reaction between sodium, carbon and oxygen atom as follows.
and it is formed by chemical reaction between sodium, carbon and oxygen atom as follows.
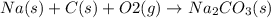
To balance this chemical equation we multiply Na by 2 and
 by
by
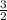 on the reactant side.
on the reactant side.
Hence, the balanced chemical equation is as follows.