Answer: Option (D) is the correct answer.
Step-by-step explanation:
A pure substance is defined as a substance that does not contain any kind of impurity and it can be a compound or an element whose composition does not vary.
For example,
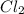 ,
,
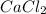 etc are all pure substances.
etc are all pure substances.
Pure substances have a fixed melting and boiling point.
A compound is defined as a substance that contains different type of atoms that are chemically combined together in a fixed ratio by mass.
An impure substance is defined as a substance that contains some kind of impurity and whose composition also varies.
A mixture is defined as a substance in which two or more substances are present but they are not chemically combined to each other. A mixture can be homogeneous or heterogeneous in nature.
Thus, we can conclude that Bromine can be classified as a pure substance.