Hello!
A) The balanced reaction is the following:
4Al(s) + 3O₂(g) → 2Al₂O₃(s)To determine the required moles of Oxygen, we can use the following conversion factor to go from moles of Aluminum Oxide to moles of Oxygen. The coefficients from the equation will be used for this conversion factor:
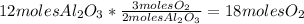
So,
18 moles of Oxygen are required to form 12 moles of Aluminum Oxide.
2) This question is similar to the above, the only difference is the mole ratio that will be used. In this case, the mole ratio would be
Al₂O₃/Al because Aluminum will be the
limiting reagent (in the lowest amount):
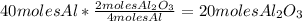
So,
20 moles of Al₂O₃ would be produced by the complete reaction of 40 moles of Al
Have a nice day!