Hello!
The neutralization equation of HCl and NaOH is the following:
HCl + NaOH → NaCl + H₂O
If 5 mL of 0,2 M HCl were required to neutralize exactly 10 mL of NaOH we can use the following equation (mole equivalency at equivalence point) to find the concentration of the base:
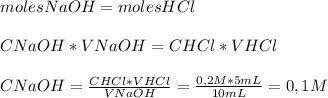
So the concentration of NaOH is
0,1 MHave a nice day!