Answer:
0.7482 g grams of copper are required .Explanation:
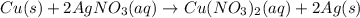
Moles of silver nitrate =
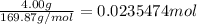
According to reaction , 2 moles of silver nitrate reacts with 1 mol of copper.
Then 0.0235 mol of silver nitrate will react with:
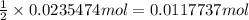 of copper
of copper
Mass of 0.0117737 mol of copper = 0.117737 mol × 63.55 g/mol = 0.7482 g
0.7482 g grams of copper are required .