Answer:
A.
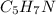
B.
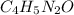
Step-by-step explanation:
Hello,
a. In this case, based on the given percentages, one computes the moles of C, H and N as follows:
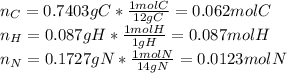
Now, we divide each element's moles by the smallest amount of moles, that is nitrogen's moles:
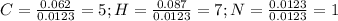
So the empirical formula is:
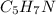
b. In this case, based on the given percentages, one computes the moles of C, H, N and O as follows:
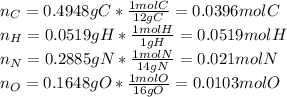
Now, we divide each element's moles by the smallest amount of moles, that is oxugen's moles:
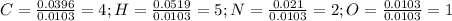
So the empirical formula is:
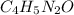
Best regards.